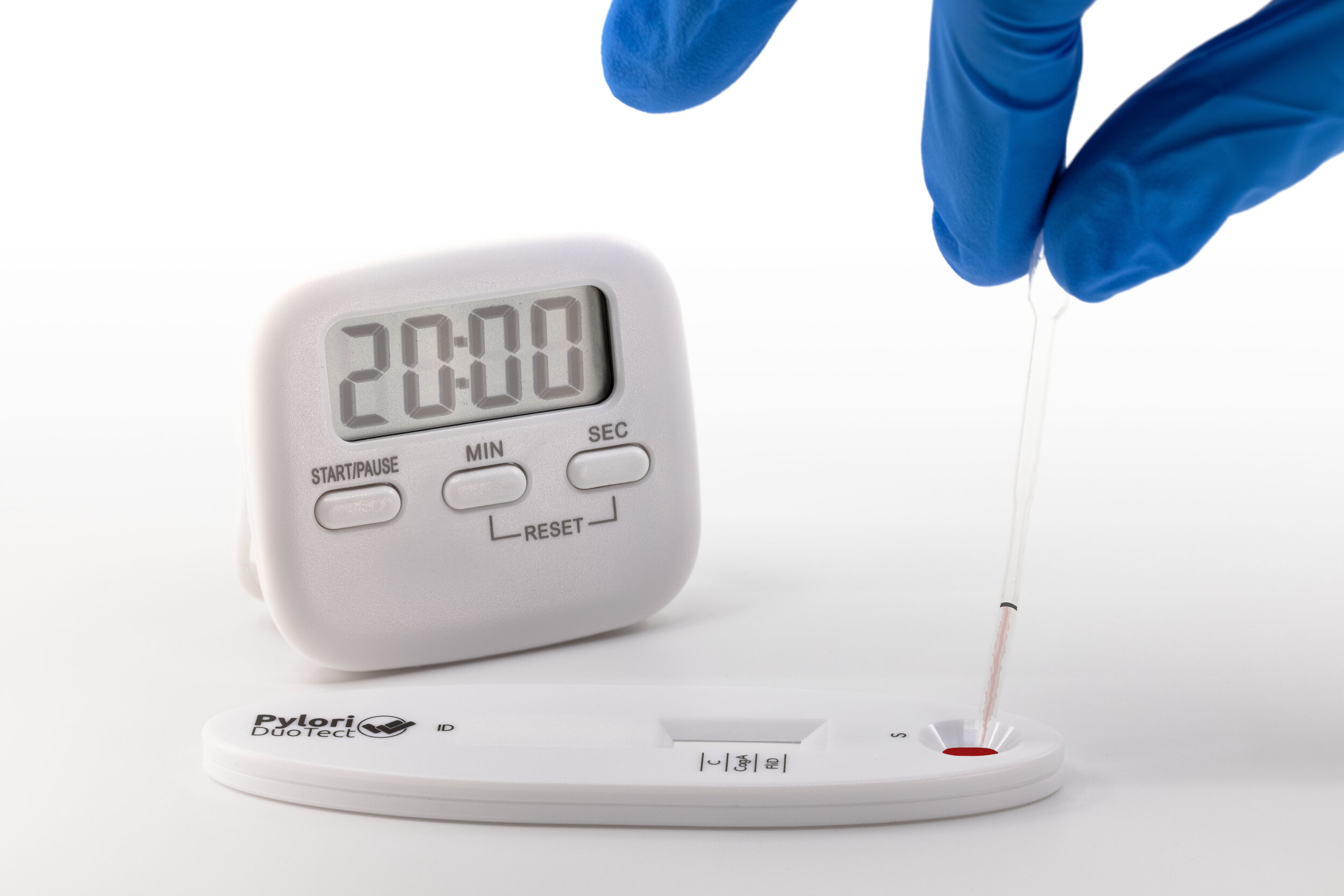
Pylori DuoTect® –
Smart, quick and Accurate
2 very sensitive and specific H. pylori infection markers guarantee the highest sensitivity
We are certainly not the first manufacturers of H. pylori rapid tests to detect the infection. But what differentiates our test from others is the approach:
Instead of using lysate, our test works with two very sensitive and very specific H. pylori antigens: FliD and CagA.
While H. pylori has a multitude of strains worldwide, the capping protein FliD is part of each H. pylori flagella, no matter where in the world it may be, and is our first marker for an H. pylori infection. Our second marker, CagA, is a well-known indicator for H. pylori infections and also serves as an additional risk factor for the development of peptic ulcers and gastric cancer.
Togehter, the two markers increase sensitivity and provide additional information about the patient’s infection and risk stratification.
Clinical performance has proven that Pylori DuoTect® can compete with current diagnostic gold standards (Sensitivity >95%) and reliably detects an H. pylori infection.
ImevaX holds the patent for the detection of FliD antibodies.
Benefits at a Glance
Time efficient
– Visual read-out within
20 minutes only
– Speeds up further examinations treatment
Cost-efficient
– No complex and expensive
instrumentation
– Low turnaround time
– Low cost per use
Convenient
– No patient preparation in advance (e.g. discontinuation of PPIs or antibiotics)
– Non-invasive with prompt results
– Intended for Point-of-Care
(POC) as well as for
laboratory use
Patented method
– Global patent for the detection of FliD antibodies. FliD is a virulence factor present in all H. pylori strains worldwide
– As accurate as diagnostic gold standards
Kit Content:
– 25 test cassettes,
each sealed in a pouch with transfer pipette
– 2 bottles of assay buffer
– 1 IFU
Product Specifications
| Assay format: | In-vitro immunochromatographic assay |
| Instrument: | Not required; visual inspection |
| Usage: | Helicobacter pylori first-line diagnostics |
| Test time: | 20 minutes |
| Sensitivity: | > 98% |
| Specificity: | > 91% |
| Accuracy: | > 93% |
| Antigen: | FliD and CagA (detection of FliD antibodies patented by ImevaX) |
| Sample material: | Capillary or venous whole blood, serum or plasma |
| Sample volume: | Whole blood: 2 drops Serum/Plasma: 1 drop |
| Risk of Cross-Reactivity: | No cross-reactivity observed |
| Kit Set-Up: | 25 individual pouches / kit |
| Shelf-Life: | 24 months |